COVID-19 CPR Solution
Updated 11 April 2020
MCC-E The Best Designed Mechanical Chest Compression Device for CPR in COVID-19 Pandemic
Challenges
Since the outbreak of COVID-19, health-care workers all around the world have worked desperately to save their patients. Nevertheless, this unprecedented new virus has seriously traumatized the public health system in all countries. Medical protective equipment and ventilators are in urgent need everywhere. One severe challenge is to carry out high quality resuscitation in saving COVID-19 patients fall into cardiac arrest.
Scientific Advice
Expert consensus on cardiopulmonary resuscitation in adult patients with novel coronavirus pneumonia suspected or confirmed in hospital[1], which was pre-published on Chinese Journal of Emergency Medicine on March 11th, put highlighted recommendation on Mechanical CPR Device. On March 31st, an Adult ALS Algorithm for The Management of Cardiac Arrest for Suspected and Confirmed COVID-19 Patients [2]was produced by Resuscitation Council UK, which also emphasized the importance of Mechanical CPR Device to facilitate transfer and treatment. Interim Guidance for Basic and Advanced Life Support in Adults, Children, and Neonates With Suspected or Confirmed COVID-19[3], released by AHA on April 9th, updated general principles for resuscitation in suspected and confirmed COVID-19 patients. One of the principles recommends replace manual chest compression with Mechanical CPR Device for adults and adolescents who meet height and weight criteria to reduce provider exposure to COVID-19. Undoubtedly, it not only protects health-care workers but also improves CPR quality.
[1] http://www.cem.org.cn/zine/content/id/9181/flag/0/zid/263 [2] https://www.resus.org.uk/media/statements/resuscitation-council-uk-statements-on-covid-19-coronavirus-cpr-and-resuscitation/covid-healthcare-resources/?utm_source=tw_date=270320 [3] https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.120.047463
COVID-19 CPR Solution
MCC-E is the best designed Mechanical CPR Device for coding patients with COVID-19.
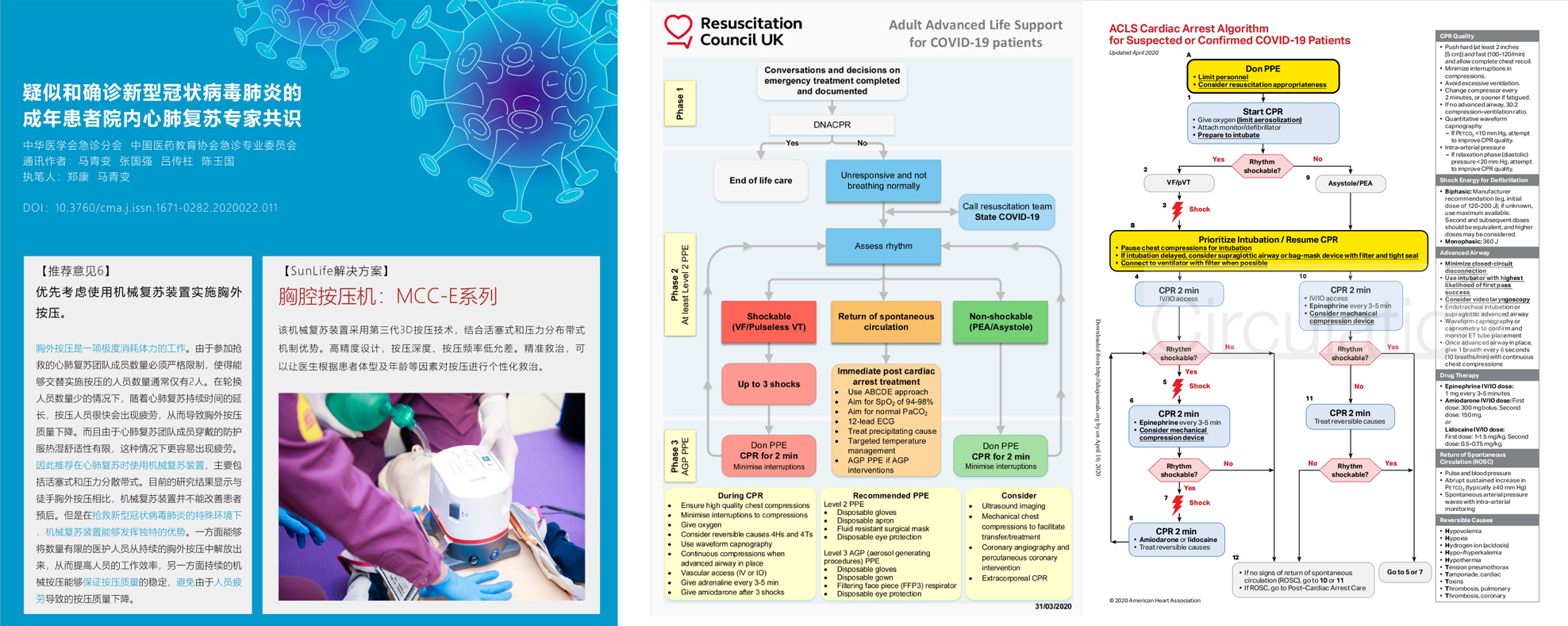
1.NMPA & CE Certifications
China Ministry of Commerce, General Administration of Customs and National Medical Products Administration have announced that starting April 1st, medical supply for export concerning coronavirus should have NMPA certification and should meet the requirements of quality standards in importing country. MCC-E is the only CPR device made in China that has both China NMPA and CE Certification.

2.China Experience
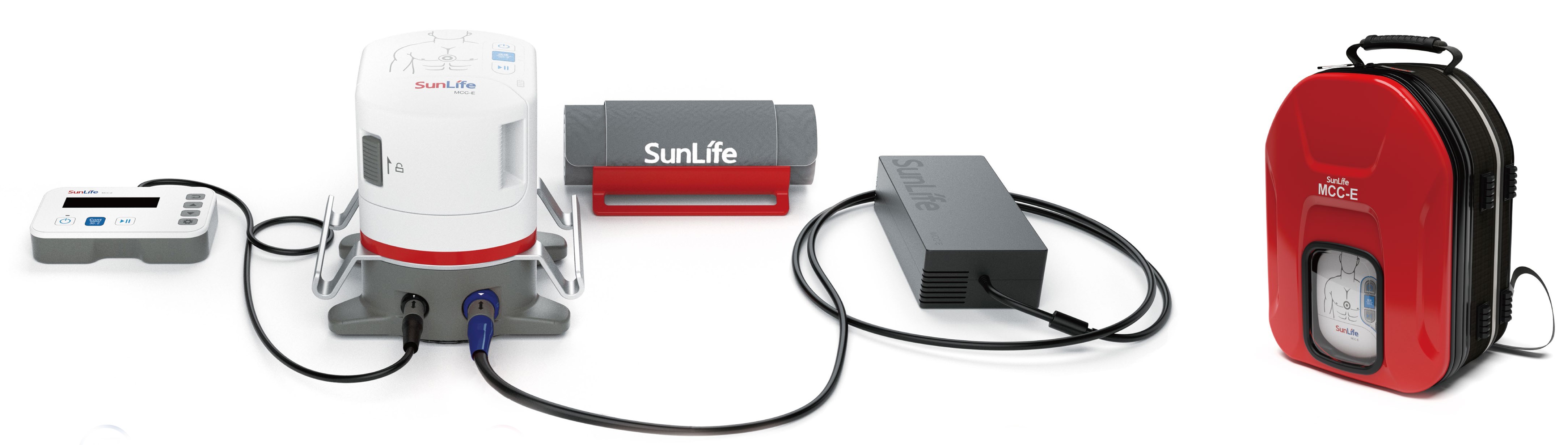
MCC-E has been widely used in the ICU and Ambulances battling COVID-19 in China in the past two months.
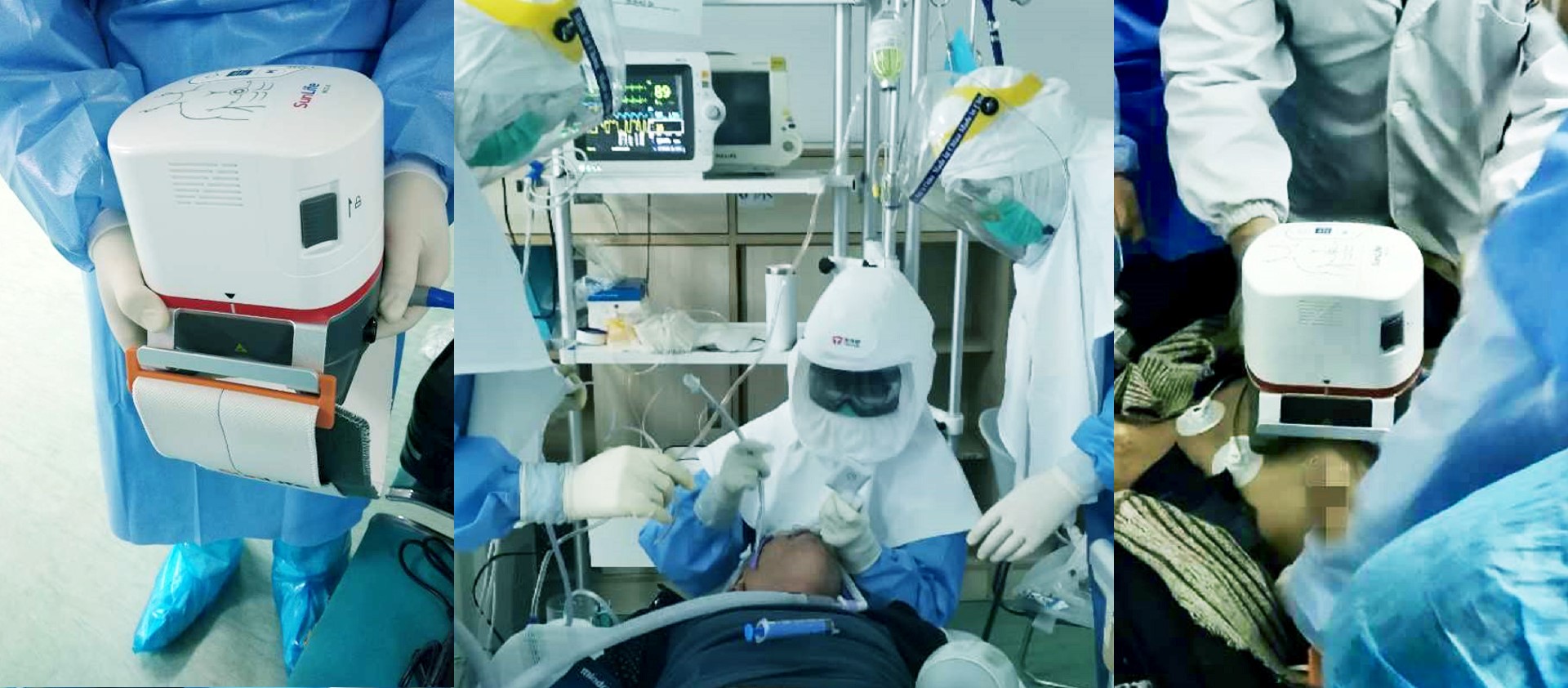
3.Weil 3D Compression Technology
MCC-E uses “Weil 3D Compression Technology”, which originated from the world renown CPR institute, the Weil Institute. Combining the advantages of Piston CPR and Load-distributing CPR, Weil 3D Compression Technology increases the positive pressure and the negative pressure by intensify the deformation of the chest, which in turn generates more blood perfusion and decreases the risk of fracture.
4.Washable and disposable accessories
Patients get in close contact with compression pad and torso restraint, two accessories of MCC-E. These accessories are wash cleaned. The affordable price of these accessories also make them disposable in case of COVID-19 contamination.
5.Uninterrupted Power System

More Certification and Compliance: